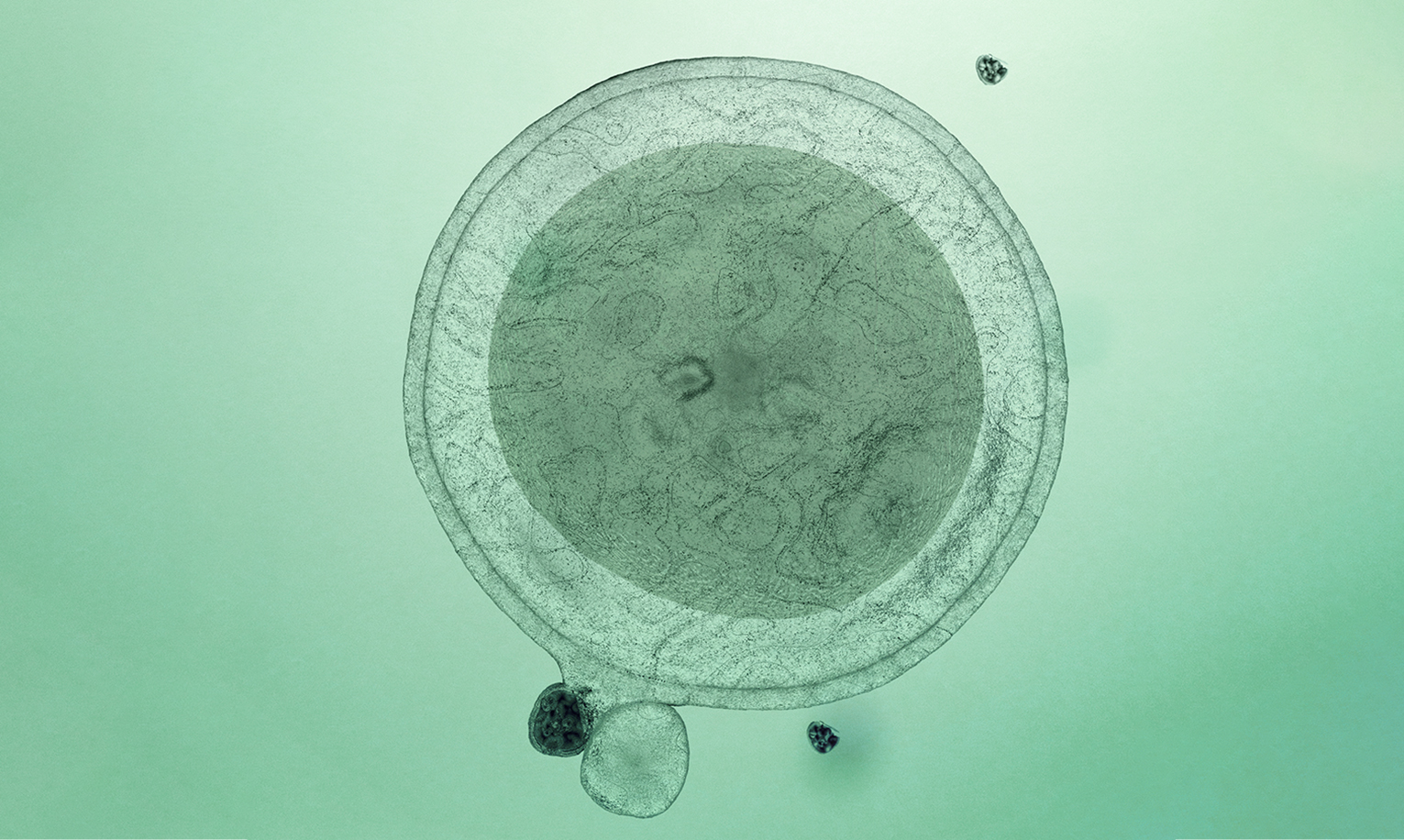
The Novo Nordisk Foundation has committed up to DKK 950 million (EUR 127 million) to establish a world-class facility for the final development steps and upscaling of cell therapies for testing in humans. The Novo Nordisk Foundation Cellerator will fill a critical gap in the Danish cell therapy ecosystem, helping to translate breakthroughs in cell therapy research into real-world treatments for people with diseases such as chronic heart failure, Parkinson’s, kidney disease, type 1 diabetes and several forms of cancer.
The facility will be located at the Technical University of Denmark (DTU) in Lyngby. It will serve public and private, national and international clients from academia, biotech and the pharmaceutical industry and is expected to be operational in 2027.
“Cell therapies have the potential to take us from treating or managing the symptoms of chronic diseases to treating the disease itself, or even curing it with a one-off procedure,” says Thomas H.R. Carlsen, CEO of the Novo Nordisk Foundation Cellerator.
“We’ve seen major advances in the laboratory in recent years, but many promising cell therapy candidates face difficulties reaching clinical trials, partly because we can’t currently develop cell therapy products in large, consistent quantities here in Denmark. I’m thrilled to be heading an initiative that will change this and provide hope to people living with chronic diseases.”
Consistent and scalable cell therapy production
Cell therapies involve the transplantation of living cells into patients to treat diseases. The primary activities at the Novo Nordisk Foundation Cellerator will be further developing cell therapies that have already been tested successfully in animals, and manufacturing these consistently and at scale for early clinical trials.
The facility will support several cell therapy types – those derived from embryonic stem cells, from induced pluripotent stem cells and from adult stem cells – and provide a range of services, from process development to product GMP manufacturing, product release and regulatory support. There will be built-in flexibility with regard to therapy types and services in order to respond to changing demands in a field that is developing rapidly.
While a few hospitals in Denmark already have small-scale facilities for manufacturing cell therapy products, the Novo Nordisk Foundation Cellerator will be the first large-scale production site and the first to gather such a broad range of expertise and services under one roof.
The Cellerator will be located at DTU partly due to the university’s significant expertise in cell manufacturing and existing infrastructure.
Anders Bjarklev, President of DTU, is pleased with the upcoming facility.
“The location of the Novo Nordisk Foundation Cellerator at DTU allows for significant synergies with our research, education and innovation in health tech and biotechnology, which is happening in close collaboration with start-ups and companies,” says Bjarklev.
“The Cellerator will provide our researchers and students with a unique opportunity to translate cell technologies into cell therapy treatments. DTU is among the world’s leading universities in biotechnology and innovation, and the Cellerator is a significant element in our vision of maintaining and strengthening that position.”
Knowledge hub for Denmark and Europe
Co-creation is at the heart of the initiative, and the Novo Nordisk Foundation is already forming strong partnerships that will be crucial for success. A key partner alongside DTU is reNEW, the Novo Nordisk Foundation Center for Stem Cell Medicine, a research consortium established in 2021, spanning Denmark, Australia and the Netherlands.
Professor Melissa Little, CEO of the consortium and Executive Director of reNEW Copenhagen, is excited about this partnership.
“At reNEW, our research into stem cells is driving the development of new stem cell-based treatments to repair or replace specific cells or tissues,” says Professor Little.
“Given our major programmes in cell and gene-modified cell therapies, the construction of the Novo Nordisk Foundation Cellerator will be a major opportunity to help move from the laboratory to the clinic. This is currently a major hurdle in the field. As such, the goals of reNEW and the Cellerator align.”
Thomas H.R. Carlsen (CEO of the Novo Nordisk Foundation Cellerator) and Foundation representatives are also in dialogue with public and private stakeholders across the Danish cell therapy landscape and further afield, gathering insights and sharing learning with similar facilities in Sweden, the Netherlands, Canada, the US and elsewhere.
“We expect this facility to become the critical link here in Denmark that enables ground-breaking stem cell discoveries to be taken all the way to proof-of-concept in human chronic disease trials,” says Professor Mads Krogsgaard Thomsen, CEO of the Novo Nordisk Foundation. “Partnering with academic, clinical and biotech scientists in the field is key to succeeding with future curative therapies.
“And we’re setting our sights even higher too. We believe the Novo Nordisk Foundation Cellerator can be a leading player in Europe and a model for similar facilities around the world, helping Denmark to punch above its weight in the search for cures to some of the world’s most serious chronic diseases.”
About the Novo Nordisk Foundation Cellerator
The Cellerator will be established as a limited liability company fully owned and funded by the Novo Nordisk Foundation, and operated as an independent philanthropic initiative.
The physical premises hosting the operations will be a newly established process development and Good Manufacturing Practice facility designed to meet the specific needs of the Cellerator services.
Construction is expected to begin in summer 2024, with the facility operational in 2027.
Read more about the Novo Nordisk Foundation Cellerator here.
Watch this video to learn more about why this initiative is needed.